PrEsentation
TRATO-TLV group designs, manufactures and commercialises lighting products and hospital equipments in the world.
Its workforce today is 250 employees with multiple skills and the company has a fleet of high technology machines. For nearly 40 years, TLV has been developing lighting and hospital equipment solutions that provide quality, comfort, safety and budget control for care teams in private and public care facilities around the world. TLV offers the most complete range of the market, implementing the most advanced technologies. The many models equipped with LEDs contribute to sustainable development thanks to the long life and high efficiency of these sources.

HISTORY

of expertise

employees

annual production of luminaires

%
export turnover

%
turnover dedicated to R&D
DESIGN OFFICE
The design office, made up of engineers and technicians, studies and develops the products as a whole, from the specifications to the serial production, through the validation and prototyping phases. We offer tailor-made lighting solutions for the retail sector. Our customers can customize lighting products that match their image and concept to make it unique. The areas of expertise of our design office remain varied and provide the ability to study parts that will be made of aluminum extrusion or plastic, plastic injection, aluminum shell molding and other manufacturing process. Our exclusive organization, in terms of design and production, ensures you a quality of work and finishes in the greatest respect of the deadlines.
INDUSTRIAL EQUIPMENT
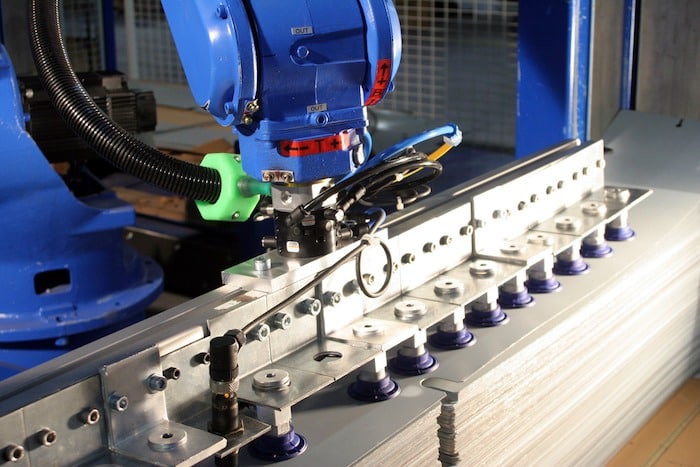
The production tool has three industrial sites with a total area of 20 000 m² located in Lille. It consists of the latest generation of machines: – Center machining CNC – Folding robots – Cutting machine water jet CNC – Chain liquid paint workshop fully automated assembly – Assembly-cabling workshop
QUALITY AND CSR
QUALITY POLICY
TLV is involved in a continual improvement process via its quality management system. This is based mainly on the following standards: – ISO 9001: Quality management systems – requirements – ISO 13485: Quality management system requirements for medical devices.
– REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 concerning medical devices (https://www.dropbox.com/scl/fi/0lx8xyeo3ezsiz1ffr8bd/Certificat_MDR_00075-A_TLV.pdf?rlkey=m15deron4n84u2gz7a8auh6fq&st=5fzk78ww&dl=0).
CSR POLICY
As part of its development, and to better preserve future generations, the TRATO-TLV Group is committed to a Corporate Social Responsibility (CSR) policy, and has implemented a targeted and equitable approach in terms of ‘pressing on 4 components:
- Economic
- Social
- Environmental
- Ethics